Abstract
Background: Iron deficiency (ID) has negative clinical consequences on physical and cognitive abilities in adolescent and adult women. Women with heavy menstrual bleeding (HMB) are at particularly high risk for ID. Guidelines issued by the American College of Gynecology recommend obtaining a CBC and serum ferritin level to screen for ID in young women with HMB. Despite the high frequency of ID in women with HMB, there are no specific recommendations regarding screening in women with bleeding disorders (WBD), though 50% or more of this population may be affected by HMB. Information on the prevalence of ID and iron deficiency anemia in WBD is lacking. In addition, understanding of the optimal practice for iron supplementation has changed, with recent literature indicating less frequent supplementation improves iron absorption. We aimed to describe the screening practices for and management of ID in WBD through a survey of medical providers within hemophilia treatment centers (HTC).
Methods: Electronic surveys were distributed by internet mail to medical providers who treat WBD within HTCs, based on membership rosters of the Hemostasis and Thrombosis Research Society and the American Thrombosis and Hemostasis Network. Data collected included indications for ID screening, laboratory measures for ID screening, iron supplementation in patients with ID (including route of administration, dose and dose frequency) and laboratory assessment to confirm iron repletion.
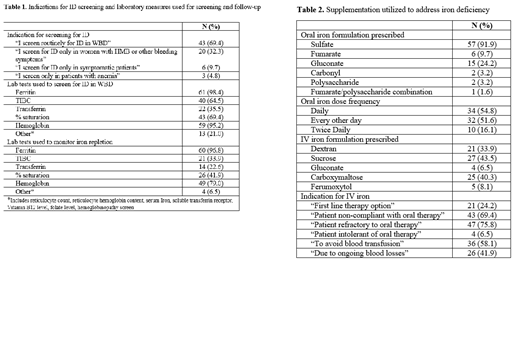
Results: Responses were received from 62 medical providers. Providers reported seeing an average of 70 WBD/year (range 1-300), with ID identified in an average of 38 WBD/year (range 2-125). Screening for ID is completed in approximately 84.5% of WBD. Screening is a part of routine practice for 69.4% of providers, only 32.3% of respondents limit screening to women with HMB or other bleeding symptoms. Over 95% of providers utilize ferritin and hemoglobin to screen for ID. When ID is identified, oral supplementation is prescribed by 96.8% of those surveyed. The most used supplement is ferrous sulfate, with daily dosing employed by 54.8% of providers and alternate day dosing employed by 51.6% of providers. Oral iron dosing varies from 15mg to 325mg elemental iron per day (or 1-6mg/kg/day with weight-based dosing). Intravenous supplementation is prescribed by 80.6% of respondents. The most common indications include refractoriness to oral iron (75.8%) and non-compliance with oral iron (69.4%). The most recommended forms of IV iron are iron sucrose (43.5%) and ferric carboxymaltose (40.3%). Repeat labs are obtained to monitor iron repletion by 96.8% of providers, typically after three months of therapy, though the laboratory measures utilized vary widely.
Discussion: All medical providers within HTCs report screening for ID in WBD, though not all WBD undergo screening. Notable variation is present in the formulation and dosing of iron supplementation used in patients with ID, as well as in follow-up practices. Given the prevalence of ID and its potential health burden, there is need for standardization of practices in screening of ID and iron replacement in WBD to promote early recognition and reduce the burden of disease in these women.
This project was supported by funding through the Health Resources and Services Administration (HRSA) of the U.S. Department of Health and Human Services (HHS) as part of HRSA H30MC2450
Ragni: Alnylam (Sanofi): Membership on an entity's Board of Directors or advisory committees; University of Pittsburgh: Research Funding; BioMarin Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Bioverativ (Sanofi): Membership on an entity's Board of Directors or advisory committees; Spark Therapeutics: Membership on an entity's Board of Directors or advisory committees; Takeda Therapeutics: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal